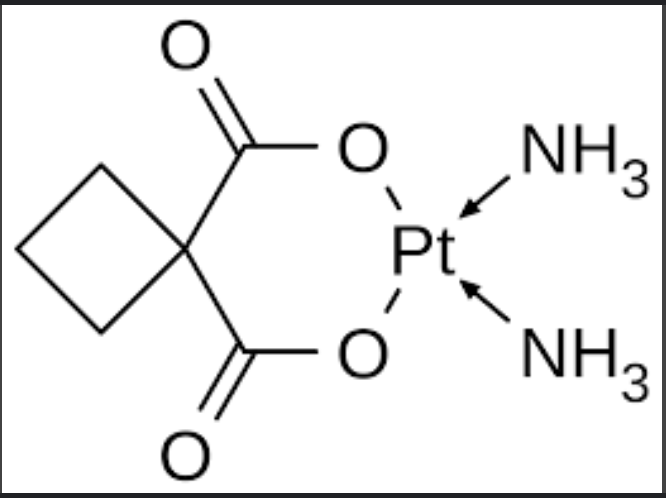
Introduction to Carboplatin
Carboplatin is a chemotherapy drug used in the treatment of various types of cancers, including ovarian, lung, and testicular cancers. It is a platinum-based compound that interferes with cancer cell division, leading to cell death. Carboplatin has become a vital part of oncology treatment regimens due to its efficacy and relatively lower toxicity compared to its predecessor, cisplatin. In the UK, carboplatin is widely used as a first-line treatment in combination with other chemotherapy agents, providing essential care for cancer patients across the country
Naprod Life Sciences: Providing EU GMP Approved Manufacturing Facilities for Cancer Treatment Drugs in European Markets
Naprod Life Sciences is an established EU GMP-certified manufacturer specializing in producing high-quality oncology medications such as carboplatin. We provide comprehensive manufacturing solutions for pharmaceutical companies in the UK through our CDMO and CMO services. Our expertise in oncology drug production ensures that healthcare providers in the UK have access to reliable, safe, and effective chemotherapy treatments, including carboplatin, that adhere to stringent European regulatory standards.
Through collaborative partnerships and a focus on continuous innovation, Naprod Life Sciences plays a pivotal role in delivering effective cancer treatments to the UK market, ultimately helping to improve patient care.
Manufacturing Excellence and Quality Assurance
Naprod Life Sciences operates state-of-the-art manufacturing facilities designed to produce high-quality chemotherapy drugs like carboplatin. Our production process is tightly regulated, with strict adherence to EU GMP guidelines. From raw material selection to final product packaging, every step of carboplatin production is carefully controlled to ensure the highest level of safety and efficacy.
TNaprod’s experience in manufacturing and supplying oncology medications globally demonstrates our capability to serve the UK market effectively. By maintaining compliance with all regional regulatory standards, we are confident in our ability to deliver carboplatin that meets the needs of UK healthcare providers.
Technological Advancements
One of the key factors that set Naprod apart in the pharmaceutical industry is its investment in technological advancements. The company employs cutting-edge technology to enhance the production and quality control processes. This includes the use of automated systems for precise formulation and packaging, as well as advanced analytical techniques for quality testing.
By leveraging these technologies, Naprod Life Sciences can produce Cisplatin that not only meets but often exceeds the quality standards set by regulatory authorities. This commitment to technological excellence ensures that patients receive the best possible treatment outcomes.
Ethical Practices and Compliance
Naprod Life Sciences prides itself on its ethical manufacturing practices. The company adheres to strict ethical guidelines in all aspects of its operations, from sourcing raw materials to the final distribution of its products. This ethical approach is reflected in the company's compliance with international standards and regulations, ensuring that all products, including Cisplatin, are safe, effective, and ethically produced.
The company’s dedication to ethical practices also extends to its corporate social responsibility initiatives. Naprod actively engages in various programs aimed at improving healthcare access and outcomes for underserved communities. This commitment to social responsibility enhances the company’s reputation and trustworthiness in the global market.
Serving Global Markets: India, UK, and Europe
Naprod Life Sciences has strategically positioned itself to serve the needs of the India, UK, and European markets. Each of these markets has unique regulatory requirements and healthcare needs, and Naprod has tailored its operations to meet these specific demands.
UK and Europe: In the UK and Europe, Naprod’s Carboplatin production adheres to the stringent standards set by European regulatory body, EU GMP. The company’s focus on quality and compliance has enabled it to establish a strong presence in these markets, with the ability to supply products through the previously mentioned CMO or out-licensing module.